 October 10, 2009 - The U.S. Food and Drug Administration (FDA) on Thursday, October 8th will tell a federal judge in Kentucky that ordering the agency to delay enforcing new tobacco laws will have "devastating consequences" on public health.
October 10, 2009 - The U.S. Food and Drug Administration (FDA) on Thursday, October 8th will tell a federal judge in Kentucky that ordering the agency to delay enforcing new tobacco laws will have "devastating consequences" on public health. U.S. District Court Western District of Kentucky - Bowling Green - a preliminary hearing was on the court calendar for October 8, 2009 from 11-12pm 1:09CV-117 Commonwealth Brands v USA, Judge Joseph H. McKinley, Jr. presiding. Judge McKinley was appointed by President Bill Clinton and began active service on August 14, 1995. Susan Ivey, Chiarman and CEO Reynolds American: On October 8, the court heard a motion to stay the restrictions on modified risk communication. October 22nd - we are waiting for the judges’ decision on that request.
U.S. District Court Western District of Kentucky - Bowling Green - a preliminary hearing was on the court calendar for October 8, 2009 from 11-12pm 1:09CV-117 Commonwealth Brands v USA, Judge Joseph H. McKinley, Jr. presiding. Judge McKinley was appointed by President Bill Clinton and began active service on August 14, 1995. Susan Ivey, Chiarman and CEO Reynolds American: On October 8, the court heard a motion to stay the restrictions on modified risk communication. October 22nd - we are waiting for the judges’ decision on that request.
Eleven public health and consumer advocacy groups on Wednesday, October 1st also asked the federal court (UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF KENTUCKY BOWLING GREEN DIVISION) to reject the lawsuit, saying the provisions are narrowly tailored to satisfy First Amendment constitutional requirements and is designed to end "decades of false health claims that have misled millions of smokers." FDA deflects challenge from Reynolds, Lorillard, others..)
The FDA is facing a challenge to its new tobacco powers, signed into law in June, from tobacco companies including Camel cigarette maker Reynolds American Inc. (RAI) and Commonwealth Brands Inc. The companies say the law imposes unprecedented restrictions on their First Amendment rights and want a federal court in Bowling Green, Ky., to order a preliminary injunction to stop enforcement of certain provisions in the regulations.
R.J. Reynolds, Lorillard, others file suit claiming law restricts communication...
A judge for the District Court for the Western District of Kentucky is holding a hearing on the preliminary injunction request and could soon decide whether to grant the injunction.
The law restricts tobacco companies from using color in most ads, bars them from saying certain products are less risky than others and stops them from selling tobacco products in combination with other items, such as soda and mouthwash.
"It is crucial to the public health that tobacco products not be marketed as reduced-risk products unless they will, in fact, reduce risks," the FDA said in a brief filed with the court.
The injunction request relates only to the restrictions on marketing tobacco products with other consumer items, and restrictions against advertising that a tobacco product is less risky than other tobacco product. Restrictions on color in ads don't go into effect until June 2010.
The companies want to be able to make claims in ads and on boxes that certain tobacco products contain smaller amounts of harmful ingredients, such as being low in tar, and are, therefore, less risky than other tobacco products.
This issue is important to companies that make smokeless tobacco products. Reynolds, for instance, makes Camel Snus, a type of tobacco that comes in a pouch in flavors like "frost" and "mellow."
In their briefing documents, the companies argue that such information is truthful and should be given to consumers.
The FDA says such information gives consumers the "mistaken belief" that the products are safe to use. The agency will allow companies to make such claims only after they prove the product does reduce a consumers risk for tobacco- related diseases. That appears to be a high hurdle. The agency notes that medical devices and prescription drugs must go through a rigorous review process before they can be sold to treat or reduce the risk of disease.
The companies say they aren't completely against the FDA authority to regulate tobacco, and that they support restrictions in marketing and advertising to children.
While Lorillard Inc. (LO) is a party to the overall lawsuit challenging the advertising restrictions imposed by the law, it isn't a party to the preliminary injunction, according to a company spokesman. The company says it didn't join the request for injunction because it doesn't intend to market tobacco products with claims that they are less risky than other tobacco products.
Reference: UPDATE: FDA Says Delaying Tobacco Authority Will Harm Public by Jared A. Favole, Dow Jones Newswires, 10/8/2009.
Read more...
Bringing the World of Tobacco Control closer together..
FDA says delaying tobacco authority will harm public..
Croatia - lifted smoking ban in bars and cafes..
 October 10, 2009 - Croatia has lifted a smoking ban on bars and cafes. The Croatian government moved on Thursday, September 10th to loosen a controversial public smoking ban.
October 10, 2009 - Croatia has lifted a smoking ban on bars and cafes. The Croatian government moved on Thursday, September 10th to loosen a controversial public smoking ban.
On April 1, Croatia banned indoors smoking in all public places, and bar owners say the restriction has halved their profits and forced many of them to close. Croatia began enforcing a ban on smoking in most indoor areas on Wednesday, May 6, 2009.
About a third of Croatia's 4.5 million people are believed to be smokers.
So the government announced Friday, October 9th that it will ease the ban, allowing spacious cafes and bars to have separate smoking areas, while smaller ones can decide whether to allow smoking or not. Smoking places will, however, have to install extensive ventilation systems. The owners are given six months to adapt.
In the European Union, 12 countries have, or are planning, some kind of ban on smoking in public places.
Reference: Smokers in Croatia go back in bars as tobacco ban lifted - temporarily by THE ASSOCIATED PRESS, 10/9/2009.
Croatia - related news briefs:
Croatia - backs down (loosens) smoking ban..;
Croatian government gives in loosens public smoking ban..;
Croatian coffee shop owners looking for break from smoking ban...
Croatia - adjusting to new smoking ban..;
Croatia begins enforcing smoking ban on May 6, 2009..;
Croatia cigarette prices to go up April 1, 2009...;
Croatia - ban in smoking in public places goes in effect..,
Croatia - A ban on smoking went into effect on October 27, 2008..;
Croatia Aims at More Stringent Anti-smoking Laws...
Click on image to enlarge; Croatian Coat-of-Arms..
Read more...
Nevada - State Supreme Court upholds smoking ban, health department prepares top strengthen penalties,,
 October 9, 2009 - The Nevada Supreme Court's decision to uphold the Nevada Clean Indoor Air Act has the Nevada State Division of Health preparing to strengthen penalties for the ban on smoking in bars.
October 9, 2009 - The Nevada Supreme Court's decision to uphold the Nevada Clean Indoor Air Act has the Nevada State Division of Health preparing to strengthen penalties for the ban on smoking in bars.
On Thursday, September 24, 2009 the Nevada Supreme Court upheld the constitutionality of civil enforcement of Nevada's indoor smoking ban. In a 34-page opinion issued Thursday, September 24th justices said a lower court properly found criminal enforcement of the 2006 voter-approved law was unconstitutional. That provision has already been severed from the statute.
But several Las Vegas businesses appealed the civil provisions, arguing the law violates equal protection guarantees because it exempts gambling areas in large casinos, as well as stand-along taverns and strip clubs.
A five-member majority rejected those arguments, saying the exemptions are proper because minors are prohibited from gambling areas in casinos, unlike in smaller bars and restaurants where slot machines are "incidental" to their overall business.
Background: Faced with competing initiatives on smoking bans in public places, Nevadans voted (over 54% of the vote) on November 7, 2006 to enact the Nevada Clean Indoor Air Act in order to protect themselves and their families from the dangers of secondhand smoke. This ban became a statewide law on December 8, 2006. According to the state law, Nevada's cities and towns are allowed to strengthen smokefree policy further at the local level.
The state Supreme Court dealt a blow to anti-smoking forces Monday, 4/13/2009 when it declined to stop lawmakers from weakening Nevada's smoking ban.
Shortly after the September 24th Supreme Court ruling, health authorities began writing a "working draft" of proposed regulations for the Southern Nevada Health District. A copy of the draft rules obtained by the Business Press calls for penalties as severe as health permit revocation for taverns that fail to enforce the smoking ban. Other proposed rules include a requirement for a "proprietor" of any "indoor place of employment," where smoking is prohibited, to "request" that those lighting up there "stop smoking immediately."
The draft of regulations is still a work in progress and not ready for public input, spokeswoman Martha Framsted said. "It is still a draft," she said. "It is not finalized and we are still getting input from the local health authorities, the Southern Nevada Health District, the Washoe County Health District and the Carson City Health and Human Services Division."
The state high court's decision to uphold the act as constitutional as a civil law was the latest disappointment for the Nevada Tavern Owners Association and slot-route operators. Earlier this year, the Nevada Legislature failed to pass a bill that would have created an exemption to the smoking ban for adult-oriented establishments.
"I guess there is a possibility that we could file a motion for a rehearing or reconsideration with the Nevada Supreme Court," he said. Another remote option would be to file a petition to the U.S. Supreme Court, because the case involved constitutional questions of equal protection and vagueness. A decision on any such filing must be made by mid-October.
Related Las Vegas Sun articles:
Study arms smoking foes Dealers display ill effects of secondhand smoke in long-awaited results, by Liz Benston, Las Vegas Sun, 5/7/2009;
Closer look at smoking ban by Nicole Lucht, Las Vegas Sun, 5/1/2009;
Casino restaurant patrons can’t elude secondhand smoke
by Nicole Lucht, Las Vegas Sun, 1/16/2009;
Study finds high pollution levels in casino restaurants
UNLV testing shows 12 of 16 at levels that exceed EPA standards by Amanda Finnegan, Las Vegas Sun, 1/15/2009;
Serve food or allow smoking: Trendy spots may face choice by Liz Benston, 8/15/2007;
Smoking ban tests resourcefulness by Ed Koch, Las Vegas Sun, 7/30/2007;
Smoking ban not doing all the banning its sponsors hoped Many taverns thumb their noses at the law, which has little teeth by Mike Trask, Las Vegas Sun, 6/21/2008;
Smoking ban is ignored by many by Liz Benston, 1/8/2007.
Reference: Officials to strengthen penalties for smoking ban
Draft regulations show authorities plan to pull health permits of bars that ignore rules by Valerie Miller, Las Vegas Business Press, 10/7/2009.
Related news brief: Nevada - lawmakers may weaken smoking ban...
Read more...
Australian Health Minister - orders facebook probe..

 October 9, 2009 - Health Minister Nicola Roxon says her department is investigating reports big tobacco companies are using social networking sites like Facebook to hook young people onto cigarettes.
October 9, 2009 - Health Minister Nicola Roxon says her department is investigating reports big tobacco companies are using social networking sites like Facebook to hook young people onto cigarettes.
University of Sydney PHD student Becky Freeman told a conference in Darwin the tobacco industry is abusing the internet because the web does not have the same advertising controls as print and television.
Ms Roxon says it looks like tobacco companies are trying to get around Australia's strict regulations."I don't think it's good form for tobacco companies to be out trying to hook young people onto tobacco when we know the harm that it causes," she said.
"So we take a very dim view of these reports. But I have asked my department to get the details, find out how much this is happening and to advise me on any actions that can be taken if that's appropriate."
Reference: Roxon orders facebook probe, ABC (Australian Broadcasting Corporation), 10/7/2009.
Related news briefs:
Social networking sites marketing tobacco products to youngsters..;
FACEBOOK - Tobacco products being promoted..;
BAT Marketing Tobacco Products Using Text Messaging...
Read more...
Canada - bill to ban flavored tobacco products gets final approval..

 October 9, 2009 - Senate passes ban on flavored tobacco products.
October 9, 2009 - Senate passes ban on flavored tobacco products.A ban on flavored tobacco products will come into effect as early as July 2010. The Cracking Down on Tobacco Marketing Aimed at Youth Act, which received royal assent (final approval) yesterday, will mean an immediate ban on advertising flavoured tobacco products in newspapers and magazines.
Flavoured cigars, known as cigarillos, blunt wraps and flavoured cigarettes, will come off store shelves as of July 5, 2010, and a ban at the manufacturer and importer level will come into effect April 6, 2010. The cigarillos, which come in a variety of candy flavors, including chocolate, grape and tropical punch, were criticized as being marketed to children and youth.
The bill passed the House of Commons unanimously in June with the backing of all three opposition parties.
Reference:
Senate passes ban on flavoured tobacco, Canwest News Service, 10/9/2009.
Related news briefs:
Canada - bill to ban flavored tobacco products gets final approval - Burley Tobacco..;
Canadian bill to ban flavored tobacco products worries Kentucky burley growers..;
Canadian Tobacco Use Monitoring Survey - 2008..;
Canadian House of Commons passes bill to prevent production of mini-cigars or cigarillos..; Canada - Bill C-32 to amend Tobacco Products Control Act..;
Canada - federal government introduces legislation to ban flavored tobacco products..;
Canada - little cigar/cigarillos smoking declined from 2007 - 2008..;
Canadian Cancer Society calls for federal ban on flavored cigars..;
Ontario to outlaw candy flavored cigars..;
Ontario poised to ban flavored cigarillos..;
Canada: a bill introduced to snuff out drive to recruit young smokers..;
Still sucking our youngsters in...
Read more...
Canada - bill to ban flavored tobacco products gets final approval - Burley Tobacco..
 October 9, 2009 - Canada has given final approval to an anti-smoking law that Kentucky burley growers and lawmakers worry may spell an end to the market for the tobacco leaf north of the border.
October 9, 2009 - Canada has given final approval to an anti-smoking law that Kentucky burley growers and lawmakers worry may spell an end to the market for the tobacco leaf north of the border.
The bill, known as the Bill C-32 - Cracking Down on Tobacco Marketing Aimed at Youth Act, passed the Senate Social Affairs, Science and Technology Committee on a voice vote and without amendments. It was awaiting final action in the Canadian Senate. (Canadian bill to ban flavored tobacco products worries Kentucky burley growers..)
The Canadian Senate passed the legislation Tuesday, October 6th and it received “royal assent” — final approval — on Thursday, October 8th.
Rob Cunningham, senior policy analyst with the Canadian Cancer Society: “This bill is a very important advance for public health in Canada.”
The bill bans flavored tobacco products in Canada.
Burley is one of three kinds of tobacco mixed together with additives for blended tobacco. Some Kentucky lawmakers, led by Rep. Ed Whitfield, R-1st District, have written to American and Canadian officials that the bill’s ban on many of the additives used in blended tobacco effectively outlaws burley. With 85 percent of U.S. burley exported, the implications of the Canadian action and possible similar actions by other nations are enormous, the Kentuckians warned.
However, U.S. trade data show that there have been no burley exports to Canada since 2006. That year, U.S. burley valued at $221,000 was delivered to Canada. In Cunningham’s view, the burley growers are raising “a non-issue.” And even if there were exports, “the bill does not ban burley tobacco, and it does not ban any type of tobacco — it bans flavors,” Cunningham said.
Roger Quarles, president of the Burley Tobacco Growers Cooperative Association, based in Lexington, Ky., said the Canadian government’s approval of the new law was expected, given the little opposition to it in the Canadian Parliament. “We’re disappointed that the government in Canada has failed to hear what we think is very logical reasoning here,” Quarles said. “That potential market for Kentucky growers will be eliminated in Canada.” He added: “We’ve always had a fear this might expand to other markets.”
While trade data may show no burley exports to Canada since 2006, that does not mean the tobacco didn’t get into the country in the form of a blend used in making cigarettes, Quarles said. And American-blend cigarettes with burley are sold in Canada, he said.
Advertisement
Cunningham said Canadians don’t care much for the American-blended cigarettes, pointing out that they constitute only 0.8 percent of the total market.
Whitfield spokeswoman Kristin Walker said the congressman remains concerned about the potential impact of the new Canadian law on Kentucky tobacco farmers.
“The issue at hand here is really the dangerous precedent this law sets internationally,” she said in an e-mail. “While burley tobacco exports to Canada in recent years have been relatively low, worldwide the U.S. exports approximately 165 million pounds of burley tobacco annually.”
Walker: “In addition, laws such as this could impact all tobacco production. Every year the U.S. exports 624 million pounds of tobacco. If every country passed laws like Canada’s, American tobacco farmers would be severely hurt.”
Rep. Whitfield is exploring ways to pressure the U.S. and Canadian governments to address the burley issue, Walker said.
Reference: Canada enacts anti-smoking law despite burley growers’ concerns by
James R. Carroll (jcarroll@courier-journal.com), Louisville Courier-Journal, 10/8/2009.
Related news briefs:
Canadian bill to ban flavored tobacco products worries Kentucky burley growers..;
Canadian Tobacco Use Monitoring Survey - 2008..;
Canadian House of Commons passes bill to prevent production of mini-cigars or cigarillos..; Canada - Bill C-32 to amend Tobacco Products Control Act..;
Canada - federal government introduces legislation to ban flavored tobacco products..;
Canada - little cigar/cigarillos smoking declined from 2007 - 2008..;
Canadian Cancer Society calls for federal ban on flavored cigars..;
Ontario to outlaw candy flavored cigars..;
Ontario poised to ban flavored cigarillos..;
Canada: a bill introduced to snuff out drive to recruit young smokers..;
Still sucking our youngsters in...
Read more...
RAI to webcast q3 2009 earnings conference call on October 22nd..
 October 9, 2009 - Reynolds American Inc. (RAI) will webcast its conference call following the release of third-quarter 2009 financial results on Thursday, October 22, 2009.
October 9, 2009 - Reynolds American Inc. (RAI) will webcast its conference call following the release of third-quarter 2009 financial results on Thursday, October 22, 2009.
During the call, members of the RA management team will discuss RAI's results for the third quarter 2009.
Announcement: Winston-Salem, NC - Oct.8, 2009, Reynolds American Inc., P.O. Box 2990, Winston Salem, NC 27102-2990..
For more information..
Read more...
United Kingdom - limiting access to cigarette vending machines not possible..
 October 8, 2009 -
October 8, 2009 -  British Heart Foundation (BHF) has repeated its calls for a ban on cigarette vending machines prior to a parliamentary debate on new ways to restrict the sale of tobacco. BHF says the machines make it too easy for young people to buy tobacco underage.
British Heart Foundation (BHF) has repeated its calls for a ban on cigarette vending machines prior to a parliamentary debate on new ways to restrict the sale of tobacco. BHF says the machines make it too easy for young people to buy tobacco underage.
As part of the Health Bill, which will be debated in Parliament on Monday, the Government has proposed that the cigarette vending machines should be age restricted, for example by a landlord or shopkeeper granting use via remote control. But the BHF published research which suggested nearly two-thirds of licensees (63%) felt this would be impossible in busy periods.
Half of the 300 pub bosses surveyed said they earned £500 or less from the machines per year, and 63% felt removing them would have no impact on their business.
Chief executive of the BHF Peter Hollins said: "The Government's proposals are unworkable and unrealistic. The message from the pub industry is loud and clear, they can't make these proposals work and the loose change they make from these machines isn't worth the hassle of keeping them.
"The only people with a real interest in vending machines are the tobacco industry. Every year young people start a life time's addiction on cigarettes by buying them from a vending machine. The Government needs to be braver and put the interests of children ahead of a commercial lobby."
According to the charity, last year 12% of children and young people in England who smoked regularly usually bought cigarettes from machines.
Mr. Hollins: "Smoking is one of the biggest avoidable causes of death and disease in the country. Yet we continue to allow vending machines which undermine the restrictions already in place, and allow children pick up an addiction they take into adulthood."
BHF says the results of its surveys show that the health conscious move would not have an adverse impact on the pub trade, with four out of five landlords claiming the revenue from the automated devices doesn't amount to much.
Jayne Murray, Public Affairs & Communications Manager at BHF NI said: "The UK Government's proposals outlined in the Bill are unworkable and unrealistic.
"The message from the pub industry is loud and clear, they can't make these proposals work and the loose change they make from these machines isn't worth the hassle of keeping them.
"The only people with a real interest in vending machines are the tobacco industry. Every year young people start a lifetime's addiction to cigarettes by buying them from a vending machine. Both the UK Government and the Northern Ireland Assembly needs to be braver and put the interests of children ahead of a commercial lobby."
For Northern Ireland BHF estimates that around 850 regular smokers aged between 11 and 15 access cigarettes from vending machines.
Survey results have showed that 70 percent of adults in the UK back proposals to protect children from tobacco by putting it out of sight in shops and 76 percent support abolishing cigarette vending machines.
Reference: Pubs ready to ditch cigarette machines/a>, Belfast Telegraph, 10/8/2009; http://uk.news.yahoo.com/21/20091008/tuk-call-for-ban-on-cigarette-machines-6323e80.html, Press Association, 10/8/2009.
Some related news briefs:
UK - Richard Branson makes high-profile plea for tobacco control measures to protect children..;
Children - exposed to cigarette smoke in cars have greater chance of respiratory distress..;
England - ban on public smoking results in a fall in heart attack by 10%..;
Updated - England - tabacco display ban - the Lords got it right..;
United Kingdom survey results adults back proposals to protect children from tobacco..;
UK - House of Lords -debate on tobacco plain packaging..
England, House of Lords votes to ban shop tobacco dislays and restrict vending machine use..;
United Kingdom to consider plain tobacco product packaging...
UK - Strategies to be implemented to prevent underage tobacco use..;
ASH calls for a debate in England on banning smoking in all cars with kids present;
British considering banning logos on cigarette packs, other measures...
Read more...
R.J. Reynolds - names like 'Light' 'Ultra-Light' being replaced with colors to invoke feelings of smoothness and health..
***Click to enlarge:
October 8, 2009 - We reported back in June 7, 2009 that some cigarette makers have started to change the names of some of their cigarettes, e.g., R.J. Reynolds investment (growth) brand Pall Mall. (C-store update: from Light, Ultra-lights to Colors; etc..)
Reynolds, the nation’s second-biggest cigarette company, makes no secret of itsreason for altering the packaging. Company spokesman David Howard cited both the impending federal regulation and a federal court ruling - currently on hold - that would also expunge the mild and light names. FDA tobacco regulations indicate that by July 2010, tobacco manufacturers may no longer use the terms "light," "low," and "mild" on tobacco products without an FDA order in effect. While the new law clearly bans use of
brand names that include “light’’ and “mild," it does not explicitly address color schemes. FDA spokeswoman Kathleen Quinn said her agency was aware of changes being made to cigarette packaging and intends, before the labeling ban goes into effect, to “thoroughly review the use of descriptors, including the use of color.’’
Howard: “By using designations such as colors, that makes it possible for retailers and adult tobacco consumers to clearly identify the different styles moving forward." The manufacturer used focus groups and other research to arrive at the new package designs, already evident on four company brands and coming soon to three of its best-known products, Camel, Doral, and Winston.
The cigarettes in the royal blue package aren’t Pall Mall Lights anymore. Now, they’re called Pall Mall Blues. Salem Lights, once sheathed in a kelly green box, are now cloaked in pastels and white, and known as Salem Gold Box. (It should be recalled that the Salem packaging has been a lot worse.)
A spokesman for Philip Morris USA (PM), which sells more cigarettes than any other manufacturer and has not dramatically altered packaging yet, said the company would not comment on its marketing plans. However, PM is already preparing to circumvent the tobacco regulation bill for instance seeking a trademark change for "Marlboro Snus Spice" to a more generic but still evocative trademarks such as "Marlboro Snus Snug Gold."
With the new branding, and use of hues shown to evoke feelings of smoothness and health, a leading tobacco company has revealed a subtle sales strategy for an era of unprecedented federal oversight: Let the colors speak to smokers in the same way the soon-to-be-banned words “mild,’’ “light,’’ and “ultralight’’ did.
Harvard researchers and other tobacco control specialists see in the new monikers and lighter, brighter palettes evidence that cigarette producers are intent on subverting a new law that empowers the US Food and Drug Administration to regulate tobacco companies - including a provision that as of next June 22 will banish words that promote certain cigarettes as safer. (President Obama signed bill for FDA to regulate tobacco on June 22, 2009.)
Tobacco control specialists have long harbored particular contempt for “mild’’ and “light’’ cigarettes, arguing they manipulate smokers into thinking those brands are less harmful when there’s no scientific evidence to support that claim.
R.J. Reynolds Tobacco Company denies attempting to bypass the law and says it is merely seeking to guide customers to their favorite brands. But researchers said they recognize the packaging changes as a tactic the industry has rolled out in other countries with stringent tobacco rules. Studies conducted in Canada and the United Kingdom, which both have a longer history of restricting tobacco industry marketing, found that smokers believe products labeled as “silver," “gold," or “smooth" are safer and easier to stop using than high-octane cigarettes.
“These tricks are now well-established,’’ said Stanton Glantz, a tobacco control specialist at the University of California, San Francisco. “The real question for the FDA is, are they going to let them get away with these shenanigans?"
While the Salem name change is only weeks old, the pack has been evolving for two years - during the same period when Congress was moving to clamp down on the industry. In 2007, according to the company spokesman, Reynolds began softening the dark green packaging for its light and ultralight Salems, opting for “a brighter, more vibrant color.’’
David Hammond, a tobacco control specialist at the University of Waterloo in Canada, has tracked tobacco branding practices around the world. “Often, they’ll change one element at a time - they change the colors and then the words follow,’’ Hammond said. “And there’s no question the companies have known that changes in the words ‘light’ and ‘mild’ were imminent in the US." But researchers, led by Hammond, have found it’s clear in the minds of consumers what the hues connote. Shown packs of cigarettes in person and online, smokers deemed “silver’’ or “gold’’ brands as being less dangerous than regular varieties.
A coalition of five major health advocacy groups wrote to the FDA last month, urging it to be vigilant for signs the tobacco industry was adopting new, color-driven names or using symbols to circumvent the regulations.
Tobacco control specialists argue that all cigarettes should be sold in plain, standard packaging with only the most neutral references to what’s inside.
Using color to cement an image is a tried-and-true staple of the advertising world. Diet Coke, for example, resides in a gleaming silver can, starkly different from the intense red container of the original variety. And cigarette makers have long appreciated the potential to use colors to speak loudly. Industry documents released through litigation noted that lighter hues were desirable because “white is generally held to convey a clean, healthy association.’’
“You don’t need McDonald’s written under the golden arches to know it’s McDonald’s,’’ said Douglas Quintal, a marketing specialist at Emerson College. “In my estimation, that’s what the tobacco industry’s banking on, that consumers will read beyond the gold and the new naming and look at the new package and say, ‘Yeah, that’s low tar, that’s low nicotine.’’
Reference: New rules, new smoke signals Color shift called trick to subvert word ban, By Stephen Smith (stsmith@globe.com), Boston Globe, 10/8/2009.
***Pall Mall Lights = Pall Mall Blues, Pall Mall Full Flavor = Pall Mall Red, Pall Mall Ultra Light = Pall Mall Orange; Salem is a Menthol cigarette, Salem Light = Salem Gold, Salem Ultra Light = Salem Silver, Salem Full Flavor = Salem Green; Capri Violet = Capri Ultra-Light, Capri Light = Capri Magenta, Capri Menthol Light - Capri Indigo, Capri Menthol Ultra-Light = Capril Menthol Light; other R.J. Reynolds cigarettes such as Doral, Winston and Kool brands have not been changed yet..
Read more...
Juneau, Alaska - voters say yes to raising tobacco taxes..
 October 8, 2009 - Beginning January 1, 2010 the tax on cigarettes will increase to $1 a pack from 30 cents. Other tobacco products will see an increase to 45 percent from 12 percent, following voters' overwhelming passage of Proposition 2 in Tuesday's municipal elections.
October 8, 2009 - Beginning January 1, 2010 the tax on cigarettes will increase to $1 a pack from 30 cents. Other tobacco products will see an increase to 45 percent from 12 percent, following voters' overwhelming passage of Proposition 2 in Tuesday's municipal elections.
The request to raise tobacco taxes came from the Juneau affiliate of the National Council on Alcoholism and Drug Dependence, which asked the Juneau Assembly Finance Committee to consider raising taxes on tobacco products as a prevention measure for teens. Executive Director Matt Felix: Some 90 percent of people who end up smoking as adults start in their teenage years. "If you can target that group and prevent them from starting through higher prices - and research shows that does work - then taxation is the way to go to get the price up.
Smoker Paul Bennett was among those voting for the tax, telling the Juneau Empire he hopes it will help him kick the habit.
Felix: Juneau was behind the curve on taxing cigarette purchases compared to other Alaskan cities..
Comparing tobacco taxes in Alaska:
Source: National Council on Alcoholism and Drug Dependence-Juneau affiliateCity Per pack Other Tobacco Products Juneau 30 cents 12% Anchorage $1.30 45% Mat-Su $1 45% Sitka $1 45% Barrow $1 12%
Reference: Voters say yes to raising tobacco taxes, by Jeremy Hsieh, Juneau Empire, 10/7/2009.
Related Alaska news briefs - State Representative concerned tobacco companies targeting our young with SNUS..; Times are Tough Save Money Quit Smoking..; Let's have fire-safe cigarettes mandatory for all 50 States - it's a NO - Brainer..; As of 7/1/2007 Smokers in five states will take a hit to their wallets as the tax increases.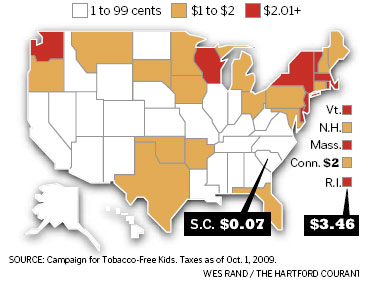
Conn. - the tax on a pack of cigarettes will be $3 a pack as of October 1, 2009..
Read more...
FDA listening sessions for tobacco stakeholders..
 October 7, 2009 - FDA held listening sessions for tobacco stakeholders in September. Thomas Briant, executive director of the National Association of Tobacco Outlets Inc. (NATO), Minneapolis, attended both of the sessions (one for manufacturers and the other for retailers, importers and distributors), as well as a media briefing.
October 7, 2009 - FDA held listening sessions for tobacco stakeholders in September. Thomas Briant, executive director of the National Association of Tobacco Outlets Inc. (NATO), Minneapolis, attended both of the sessions (one for manufacturers and the other for retailers, importers and distributors), as well as a media briefing.
While many attendees were pleased to know their concerns would be heard, they also were less than satisfied by the lack of answers. Some people were disturbed by the fact that the FDA didn't respond to commentary during the sessions, said Norm Sharp, president of the Cigar Association of America Inc., Washington. He described his own reaction as "not satisfied, but not dissatisfied."
One of the major concerns discussed was the ban on color advertising on tobacco products in retail stores. Another major concern was whether the flavored-cigarette ban includes flavored little cigars. The FDA had issued a letter to the industry September 14 that said the standard applies to "all tobacco products that meet the definition of a 'cigarette' in section 900(3) of the Act even if they are not labeled as 'cigarettes' or are labeled as cigars of some other product." Briant, however, told CSP Daily News, "Our understanding of the law is that flavored cigar products are not banned at this time, but the FDA has not provided clear guidance to clarify the issue..
Briant: "I think they truly do not understand the tobacco industry or the different tobacco products sold to consumers, and without a good underlying knowledge of these products and why they are different from one another, the FDA does not yet know how to apply the new law," he said.
The FDA also did extend a deadline for public comments from September to December. "The comment period was extended for a variety of reasons," said FDA spokesperson Kathleen Quinn. Briant: While the comments won't change the law, Briant said, "If the FDA staff takes the time to review all those comments, I think they will become educated about tobacco products and make more reasonable decisions on how to implement the law; however, that presupposes they're going to read these thousands and thousands of pages of comments." Also on deck: "Menthol will come in front of our tobacco scientific advisory committee for review," according to Quinn.
One of the concerns Briant cited about the reading of the comments was the lack of staffing at the FDA's Tobacco Products Center.
Enforcement, according to a transcript of the media briefing, provided by Briant, Catherine Lorraine, a lawyer on FDA staff, said, "I think we will be working with our enforcement team to find the most effective ways of identifying violations of this act, and we will be bringing appropriate enforcement actions when we do document violations." The FDA has also set up a toll-free number and website for the public to report violations.
According an frequently asked questions (FAQ) document, the FDA has a busy docket ahead, including:
* By January 2010, tobacco manufacturers and importers will submit information to FDA about ingredients and additives in tobacco products.
* By April 2010, FDA will reissue the 1996 regulation aimed at reducing young people's access to tobacco products and curbing the appeal of tobacco to the young.
* By July 2010, tobacco manufacturers may no longer use the terms "light," "low," and "mild" on tobacco products without an FDA order in effect.
* By July 2010, warning labels for smokeless tobacco products will be revised and strengthened.
* By October 2012, warning labels for cigarettes will be revised and strengthened.
The FDA plans to update the Frequently Asked Questions (FAQ) as questions come in to the agency..
See reference to read the complete article..
Reference: FDA Gets an Earful "Listening sessions" on tobacco regulation leave retailers with unanswered questions by Linda Abu-Shalback Zid, CSP Daily News, 10/6/2009.
Center for Tobacco Products related news briefs:
FDA Center for Tobacco Products - draft document issued..;
FDA deflects challenge from Reynolds, Lorillard, others..
FDA - began collecting fees from nations tobacco companies..;
FDA - first steps in the role of tobacco regulation..
U.S. - flavored cigarettes illegal after Wednesday, September 22, 2009..;
FDA Moves Forward on Implementation of Tobacco Law..;
Dr. Lawrence Deyton to head FDA's Tobacco Center..;
U.S.- creating the FDA's Center for Tobacco Products..
U.S. FDA posts job for new tobacco czar..;
President Obama signs bill for FDA to regulate tobacco...
Read more...
San Francisco - Michael Jordan caught smoking on public golf course..
 October 7, 2009 - San Francisco is asking basketball superstar Michael Jordan to snuff out the cigars, after he was caught on the front page of The Chronicle's Sporting Green breaking the city's ban on smoking on public golf courses.
October 7, 2009 - San Francisco is asking basketball superstar Michael Jordan to snuff out the cigars, after he was caught on the front page of The Chronicle's Sporting Green breaking the city's ban on smoking on public golf courses.
The Presidents Cup golf tournament is being held at San Francisco.
City officials sprang into action after seeing the full-color photo Tuesday of the NBA Hall of Famer enjoying a good cigar while teeing off at Harding Park's 14th hole during a Presidents Cup practice round.
In an interview with PGATour.com, Jordan was asked how many cigars he planned to smoke during this week's tournament. "Well," Jordan replied, "that depends, because I heard this is a public place, so they limit what you can smoke. I'm not even supposed to be smoking, but this was a practice round and no one said anything." If he is permitted to smoke, Jordan said, "I would say (it's) a three-cigar round. I would try to keep it at a minimum of three."
And what is the city's response? "You mean about Spare the Air Jordan?" said Recreation and Park General Manager Phil Ginsburg.
"I've already sent off an e-mail to the PGA Tour director," Ginsburg said. "It was sort of a gentle nudge reminding them that smoking is illegal and that we would appreciate their support."
Initially, public golf courses were exempt from the city's many smoking bans. However, in 2006, after much debate, the Board of Supervisors narrowly voted to apply the same rules to golf courses as other parks - including the possibility of a $100 fine for violators. "But don't expect me to ask him for it," said city attorney's spokesman Matt Dorsey.
Reference: Michael Jordan caught smoking, Phillip Matier, Andrew Ross, San Francisco Chronicle, 10/7/2009.
San Francisco related news briefs:
San Francisco - cigarettes cost a little more starting today, October 1, 2009..;
San Francisco can enforce its ban on tobacco sales in drugstores..;
San Francisco - fight about selling tobacco products in drugstores has flared up..
San Francisco - Judge threw out a Walgreens attempt to stop the ban of tobacco sales in pharmacies..;
Philip Morris appeals tobacco ban at San Francisco pharmacies;
Federal Judge Denies Bid To Stop San Francisco Pharmacy Tobacco Ban..;
Philip Morris USA request stop in San Francisco's ban on tobacco sales by pharmacies..;
San Francisco - cigarette sales rise sharply in c-stores..;
San Francisco files brief to oppose bid by PM USA to block the banning of tobacco sales in pharmacies..;
Philip Morris challenges San Francisco pharmacy tobacco ban..;
Walgreen: San Francisco’s Tobacco Ban Is Unfair..;
San Francisco - All Tobacco Products Banned in All Pharmacies..;
San Francisco critical vote - bar tobacco sales pharmacies..;
SAN FRANCISCO Ban on tobacco at drug stores sought...
Read more...
Nat Sherman Co. letter clarifying company's cigarette brands comply with the FDA regulations..
 October 7, 2009 - NEW YORK -- The Nat Sherman Co. has issued a letter clarifying which of the company's cigarette brands comply with the new U.S. Food & Drug Administration (FDA) regulations, according to the National Association of Tobacco Outlets (NATO), which published a copy of the letter in its most recent E-News Bulletin.
October 7, 2009 - NEW YORK -- The Nat Sherman Co. has issued a letter clarifying which of the company's cigarette brands comply with the new U.S. Food & Drug Administration (FDA) regulations, according to the National Association of Tobacco Outlets (NATO), which published a copy of the letter in its most recent E-News Bulletin.
The letter said:
"Regarding the FDA ban on characterizing flavors which went into effect on September 22, 2009.... The law bans both the manufacturing and sale of all flavored cigarettes (except for regular and menthol brands). This includes our Clove and Mint brand styles, but does not affect any of our other brands. In the last few weeks, we have been actively removing our Clove and Mint brands from the market place as many states have begun delisting them from their approved directories.
Consistent with our obligations as a responsible manufacturer, we have discontinued our 'A Touch of Cloves' and all our Mint brands styles. Concerning our 'Mint' brands, while many competitors use a synthetic menthol flavoring to treat the tobacco in their products, we have always remained true to our heritage as a manufacturer of only all-natural 100% additive-free cigarettes. Since menthol is derived from nature's own mint plant, our menthol-flavored cigarettes have always contained pure menthol crystals in our filters to impart a natural menthol flavor. And to differentiate our menthol products, we adopted the descriptor 'Mint.' Now, however, we have revised our packaging for these brands and changed the descriptor to 'Menthol.' Other than this slight name change, you can be assured the quality and enjoyment you and your adult customers have come to expect from these brands will continue to remain the same. Please note that both the UPC and product code will remain unchanged."
Click to read the full letter with a list of brands.
New York City-based Nat Sherman's all-natural brands of luxury cigarettes can be found in all 50 states and in more than 40 countries. It makes cigarettes, cigars and pipe tobaccos and tobacco accessories.
Reference: Nat Sherman Issues Letter on Flavors
Clarifies which of its cigarette brands comply with new FDA regulation, Convenience Store / Petroleum (CSP) Daily News, 10/5/2009.
Related news briefs: Altadis USA to sell Nat Sherman premium cigars..
Read more...
South Dakota - statewide smoking ban trial date moved to mid-November..
 October 7, 2009 - South Dakota's statewide smoking ban has now made its way to a courtroom. Both sides of the issue gathered Tuesday in Hughes County where they heard from a judge for the first time.
October 7, 2009 - South Dakota's statewide smoking ban has now made its way to a courtroom. Both sides of the issue gathered Tuesday in Hughes County where they heard from a judge for the first time.
The statewide smoking ban has been a red hot issue since state lawmakers passed the law seven months ago. One of the decisions made Tuesday by the judge is that the American Cancer Society can join the case. The organization argues that the smoking ban should be in effect now and not put to a vote because of the immediate health benefits.
"I think it is important we get back to that threshold issue of, did the legislature intend this to go into effect? Should this go into effect because of the public health preservation?" Jennifer Stalley of the American Cancer Society said.
Both sides also worked out how many petition signatures the smoking ban opponents are short of in order to refer the issue to a public vote. Larry Mann now says his cause is just 54 signatures short, and he's confident the court could review and approve enough to send the issue to the ballot box.
“We think so. Obviously, our opponents don't. But it's one of those complicated issues not knowing how a judge will rule," Mann said.
The trial date has now been moved back to mid-November, and after Tuesday's motions hearing, both sides are still confident the law is on their side. (The judge also reset the trial for Nov. 12-13, three weeks later than it had been previously scheduled. She also put off further arguments on motions until the first morning of the trial.)
"We're prepared to make a strong case that we've got a lot of signatures that ought to be included in that count," Mann said.
"I think we're confident South Dakota will eventually become smoke free. We just are working to make that happen sooner rather than later," Stalley said.
If the smoking ban does go up for a public vote, it would likely happen in November of 2010.
Reference: Cancer Society Joins Smoking Ban Battle, Keloland.com, 10/6/2009; Group will be part of smoking ban court case in November by Bob Mercer, American News Correspondent, 10/7/2009.
Developments - related news briefs:
South Dakota - trial delayed in fight to enforce smoking ban..;
South Dakota - new judge appointed in the smoking ban dispute..
South Dakota - ACS wants smoking ban passed by legislature to begin ASAP..
South Dakota - opponents of smoking ban gain a delay..;
South Dakota - petition rejected - state smoking ban to take effect..;
South Dakota - Secretary of State's Office still counting disputed signatures on the smoking ban petitions..;
South Dakota - anti-smoking leaders challenge petition..;
South Dakota - smoking ban to start July 1, 2009 may be delayed..;
South Dakota - opponents try to stop extended smoking ban..;
South Dakota - extends smoking ban effective July 1, 2009...
Read more...
U.S. Institute of Medicine: Report - Secondhand-Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence..
 October 7, 2009 - Secondhand-Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence, a new report from the Institute of Medicine, provides a comprehensive evaluation of studies exploring the impacts of smoking bans in the United States and abroad and the relationship between secondhand smoke and heart disease. Based on this review, the report offers conclusions about the effectiveness of smoke-free policies. The report will be released with a one-hour public briefing.
October 7, 2009 - Secondhand-Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence, a new report from the Institute of Medicine, provides a comprehensive evaluation of studies exploring the impacts of smoking bans in the United States and abroad and the relationship between secondhand smoke and heart disease. Based on this review, the report offers conclusions about the effectiveness of smoke-free policies. The report will be released with a one-hour public briefing.
Details:
11 a.m. to noon EDT Thursday, Oct. 15, Room 201 of the National Academies Keck Center, 500 Fifth St., N.W., Washington, D.C. Those who cannot attend may participate by conference call.
Advance copies of the report will be available to reporters only beginning at 9 a.m. Wednesday, Oct. 14. The report is embargoed until 11 a.m. EDT on Oct. 15. Reporters: To receive a copy of the report and to register to attend the briefing or to receive the call-in information, contact the Office of News and Public Information, tel. 202-334-2138, or e-mail news@nas.edu.
Reference: NEWS - from the National Academies.., 10/7/2009.
Read more...
Altria to Host Webcast of q3 2009 results on October 21st..
 October 7, 2009 - Altria Group, Inc. (Altria) (NYSE: MO) will host a live audio webcast on Wednesday, October 21, 2009 at 9:00 a.m. Eastern Time to discuss 2009 third-quarter business results. The business results will be issued by means of a press release at approximately 7:00 a.m. Eastern Time the same day. The webcast can be accessed at www.altria.com.
October 7, 2009 - Altria Group, Inc. (Altria) (NYSE: MO) will host a live audio webcast on Wednesday, October 21, 2009 at 9:00 a.m. Eastern Time to discuss 2009 third-quarter business results. The business results will be issued by means of a press release at approximately 7:00 a.m. Eastern Time the same day. The webcast can be accessed at www.altria.com.
During the webcast, Mr. Michael E. Szymanczyk, Chairman and Chief Executive Officer, and Mr. David R. Beran, Executive Vice President and Chief Financial Officer, will discuss the company's 2009 third-quarter business results and answer questions from the investment community and news media.
The webcast will be in a listen-only mode. Pre-event registration is necessary; directions are posted at www.altria.com. An archived copy of the webcast will be available until 5:00 p.m. Eastern Time on Thursday, November 19, 2009, at www.altria.com.
SOURCE: Altria Group, Inc.
Investor Relations
Altria Client Services
804-484-8222
Read more...
Social networking sites marketing tobacco products to youngsters..
 October 7, 2009 - Tobacco companies are using legal loopholes to market products on social networking sites including Facebook and MySpace targeting young smokers.
October 7, 2009 - Tobacco companies are using legal loopholes to market products on social networking sites including Facebook and MySpace targeting young smokers.
Advertising restrictions on cigarette giants are forcing companies to become savvier in the way they reach consumers.
Fan clubs and unofficial product pages endorsing Marlboro, Benson and Hedges and Lucky Strike are now appearing on social networks, and have the ability to redirect users to the product's website, The Daily Telegraph reports. Cancer Council Australia has called on the Federal Government to intervene and ensure the sites are pulled down.
Tobacco companies have denied officially setting up Facebook and Myspace pages. But experts said it was difficult controlling the stealth internet advertising with regulation a global problem. "It needs to be brought to the Government's attention because they have been very good at restricting tobacco advertising," Professor Ian Olver from the Cancer Council said. "Now we have this whole new media that can be used and needs to be looked at because it can get a huge number of people, particularly young people."
Marlboro has 5058 followers on Facebook while Benson and Hedges also uses the social
networking site. Welcome to the official Facebook Page of ***Marlboro Black Menthol... University of Sydney researcher Becky Freeman has been studying the proliferation of tobacco companies using the internet as a marketing tool. "One of the most innovative marketing strategies was by the Camel brand, which engaged the online community to help design a new packet," she said.
University of Sydney researcher Becky Freeman has been studying the proliferation of tobacco companies using the internet as a marketing tool. "One of the most innovative marketing strategies was by the Camel brand, which engaged the online community to help design a new packet," she said.
Reference: Tobacco companies using social networking to target young by Kate Sikora, The Daily Telegraph, 10/7/2009.
Related news briefs:
FACEBOOK - Tobacco products being promoted..;
BAT Marketing Tobacco Products Using Text Messaging...
***Marlboro Black Menthol was launched last August 2008 in Japan, where it has become our most successful new launch ever in this important market. It has achieved a 1.0% share of market and has enabled the Marlboro brand family to resume its growth, reaching a 10.4% market share in the first quarter of this year. Given the success of Marlboro Black Menthol in Japan, it was launched during the first quarter in Hong Kong and Indonesia. (PMI - 2009 Annual Meeting of Stockholders, May 5, 2009..)
Read more...
Nicotine Vaccine - funding for Phase 3 clinical trial..
 October 7, 2009 - A $10-million grant to Nabi Pharmaceuticals of Rockville, Md., from the National Institute on Drug Abuse will fund a Phase 3 clinical trial of a new vaccine designed to prevent relapses among smokers -- the final step before the vaccine can be approved for general use. It is the first large trial of an anti-smoking vaccine.
October 7, 2009 - A $10-million grant to Nabi Pharmaceuticals of Rockville, Md., from the National Institute on Drug Abuse will fund a Phase 3 clinical trial of a new vaccine designed to prevent relapses among smokers -- the final step before the vaccine can be approved for general use. It is the first large trial of an anti-smoking vaccine.
The vaccine, called NicVax (Nicotine Conjugate Vaccine), stimulates the production of antibodies that bind to nicotine in the bloodstream. The bound nicotine molecules are too large to enter the brain, thereby subverting the rewarding effects of the drug. Preliminary studies have shown that smokers who achieved the highest level of nicotine antibodies had higher rates of quitting smoking and longer durations of abstinence than those given placebos. The vaccine was also well tolerated, with few side effects.
Some evidence suggests that the antibodies may persist for only six to 12 months, but that may be long enough to allow smokers to get through the extremely difficult first months of withdrawal.
Smoking is the largest cause of preventable deaths in the United States, with more than 400,000 deaths directly linked to it each year.
More information..
Reference: Stimulus money will fund nicotine vaccine trial, Thomas H. Maugh II, Los Angeles Times, 9/30/2009.
Read more...
Smoking during pregnancy and postnatal environment - socioeconomic inequalities..
 October 6, 2009 - New research published in BMJ reports that addressing the problem of smoking during pregnancy may help to reduce the socioeconomic inequalities in stillbirths and infant deaths by as much as 30 to 40 percent.
October 6, 2009 - New research published in BMJ reports that addressing the problem of smoking during pregnancy may help to reduce the socioeconomic inequalities in stillbirths and infant deaths by as much as 30 to 40 percent.
Without a doubt smoking during pregnancy has been associated with stillbirth. In addition, infant deaths and smoking rates during pregnancy vary strikingly with socioeconomic position. In order to find out more, a team of researchers began the task of measuring the effects of smoking during pregnancy and on the social inequalities gap in stillbirths and infant deaths.
They assessed the records of 529,317 live singleton births and 2,699 stillbirths delivered at 24 to 44 weeks' gestation in Scotland from 1994 to 2003. Information on smoking during the pregnancy was gathered. A deprivation score was designated using postcode data from the 2001 population census.
PAPER: Contribution of smoking during pregnancy to inequalities in stillbirth and infant death in Scotland 1994-2003: retrospective population based study using hospital maternity records, Ron Gray, clinical epidemiologist1, Sandra R Bonellie, lecturer in statistics, James Chalmers, consultant in public health medicine, Ian Greer, dean, Stephen Jarvis, emeritus professor, Jennifer J Kurinczuk, reader in perinatal epidemiology, Claire Williams, statistician, BMJ 2009;339:b3754, ABSTRACT.., Full Text...
Findings showed that the most underprivileged mothers tended to be younger. They were more likely to smoke and to give birth to preterm or low birth weight babies. In the same way, the least deprived mothers were more likely to be older, non-smokers, and less likely to give birth to preterm or low birth weight babies.
The stillbirth rate increased from 3.8 per 1,000 in the least deprived group to 5.9 per 1,000 in the most deprived group. The rate of infant deaths increased from 3.2 per 1,000 in the least deprived group to 5.4 per 1,000 in the most deprived group.
In the most deprived category, stillbirths were 56 percent more likely and infant deaths were 72 percent more likely, compared with the least deprived category.
Smoking during pregnancy accounted for 38 percent of the inequality in stillbirths and 31 percent of the inequality in infant deaths. Women in the most deprived group were three times more likely to smoke during pregnancy than were those in the least deprived group.
In closing, the authors propose tackling (convince to stop) smoking during pregnancy and also reducing infants' exposure to tobacco smoke in the postnatal environment. This will help reduce stillbirths and infant deaths in general, as well as to reduce the socioeconomic inequalities in stillbirths and infant deaths possibly by as much as 30 to 40 percent.
Nevertheless, they underline that taking action on smoking on its own is doubtfully sufficient. There is a need for other measures to improve the social circumstances, social support, and health of mothers and infants.
Reference: Stillbirths And Infant Deaths Related To Smoking During Pregnancy And Socioeconomic Inequalities by Stephanie Brunner, Medical News Today, 10/2/2009.
Some pregnancy related news briefs:
Maternal Smoking - important risk factor in the development of psychotic experiences in their children..;
England - program to pay pregnant women not to smoke seems hopeful..;
Pregnant women who quit smoking before the 15th week reduce risk of premature birth and small babies..;
Pregnant women exposed to passive smoke greater chance of child will have respiratory distress..;
Cigarette Smoking During Pregnancy Alters Maternal and Fetal Thyroid Function..;
Read more...
Wales - Smoking 'costs NHS £1m (1.6m USD) each day'..

October 5, 2009 - Every year 6,000 people die in Wales as a result of smoking. New figures indicate that smoking is costing the National Health Service (NHS) in Wales more than £7m (11.128m USD) every week. (The United Kingdom is made up of England, Wales, Scotland and Northern Ireland).
A report commissioned by  Ash Wales and
Ash Wales and  British Heart Foundation Cymru reveals that smoking related diseases cost NHS Wales an estimated £386m in 2007/08. (The native (Welsh) name for the country is Cymru, which most likely meant "compatriots" in Old Welsh.)
British Heart Foundation Cymru reveals that smoking related diseases cost NHS Wales an estimated £386m in 2007/08. (The native (Welsh) name for the country is Cymru, which most likely meant "compatriots" in Old Welsh.)
Smoking accounts for around 22% of adult hospital admission costs, over £235m (345.3m USD) every year, the research said. The assembly government said tackling smoking was a priority and attitudes were changing towards smoking.
The report, being presented at an international tobacco control conference in Cardiff by Prof Ceri J Phillips of Swansea University, said £43m (63.2m USD) was also spent on GP consultations. It said that nearly a quarter of the adult population in Wales are smokers and most of these started smoking as children.
With 6,000 people in Wales dying each year as a result of smoking, the report said many more would continue to die each year, or suffer chronic long-term illnesses, if these levels were sustained.
Tanya Buchanan, of Ash Wales, said: "What this report doesn't include is the huge cost to the economy of Wales, for example more than £23m (33.8m USD) from lost productivity through smoking related sickness absences and £6m (8.8m USD) from smoking related fires.
THE REPORT'S MAIN FINDINGS
Smoking cost NHS Wales an estimated £386m in 2007/08; equivalent to £129 per head and 7% of total healthcare expenditure in Wales.
£235.6m spent on hospital admissions (22% of total)
£43.1m spent on GP consultations (13%)
£21.5m spent on outpatient attendances (6%)
£6.2m spent on practice nurse consultations (12%)
£79.3m spent on prescriptions (14%)
Source: Ash Wales and British Heart Foundation Cymru
"Not to mention the emotional cost of family members seeing their loved ones suffer daily from smoking related illnesses."
Ms Buchanan said that the report should not be used to demonise smokers but to prompt a move towards a more proactive health service, promoting and protecting people's health throughout their lives. "We urge the Welsh Assembly Government to act now and implement a comprehensive, and fully funded, tobacco control strategy for Wales, in line with other parts of the UK," she said.
Delyth Lloyd, public affairs manager for British Heart Foundation Cymru, added: "The findings of this report should be of real concern to all who are involved in public health policy and decision making in Wales."
A Welsh Assembly Government spokesman said it had made tackling smoking a priority and there were encouraging signs that attitudes towards smoking in Wales were changing. "Research we have commissioned shows that the smoking ban, introduced in April 2007, has had a significant impact on smoking habits, with people reporting that they're smoking less and thinking more about quitting," said the spokesman.
Schemes such as Smokebugs and Assist, which aim to prevent young people trying tobacco, were also starting to pay off, he said.
"The number of 15 to 16-year-old boys smoking has dropped from 21% in 1998 to 12% in 2006, while smoking among girls in that age group has fallen from 29% in 1998 to 23% in 2006."
Reference: Smoking 'costs NHS £1m each day', BBC, 10/5/2009.
Click on image of the Wales Coat of Arms to enlarge..
Read more...
New Jersey assemblywoman wants to limit electronic (e) cigarettes..
 October 6, 2009 - New Jersey State Assemblywoman Connie Wagner (D-Bergen) says she wants to limit electronic cigarettes like the real thing.
October 6, 2009 - New Jersey State Assemblywoman Connie Wagner (D-Bergen) says she wants to limit electronic cigarettes like the real thing.
State Assemblywoman Connie Wagner (D-Begen) said she is concerned that e-cigarettes are being marketed to children because they offer flavors like chocolate, banana and strawberry.
The Democrat from Paramus intends to introduce a bill in the Legislature subjecting them to the same restrictions as pipes and regular cigarettes.
E-cigarettes look like the real thing but don't contain tobacco. They employ a metal tube with a battery that heats up a nicotine solution. Users breathe in the resulting vapor.
Wagner's bill would prohibit their use in public places and workplaces.
The FDA “has already declared that electronic cigarettes are subject to FDA approval as a drug or medical device, much like nicotine patches, gum and inhalers — and therefore they are illegal until they are cleared,” and has stated that electronic cigarettes are “unapproved new drugs and/or misbranded drugs or devices,” and appear “to be a combination drug-device product that requires pre-approval, registration and listing with FDA.” ("Smoking Everywhere", an electronic cigarettes retailer sued..)
Reference: N.J. assemblywoman seeks sale restrictions for e-cigarettes, by The Associated Press, 10/6/2009.
Read more...
Australia - heavy tobacco use killing Aborigines..

 October 6, 2009 - Smoking is a "scourge" that is killing thousands of indigenous Australians every year, Aboriginal and Torres Strait Islander Social Justice Commissioner Tom Calma says. (The term "Aboriginal" has traditionally been applied to indigenous inhabitants (original inhabitants of the Australian continent and nearby islands),of mainland Australia, Tasmania, and some of the other adjacent islands.)
October 6, 2009 - Smoking is a "scourge" that is killing thousands of indigenous Australians every year, Aboriginal and Torres Strait Islander Social Justice Commissioner Tom Calma says. (The term "Aboriginal" has traditionally been applied to indigenous inhabitants (original inhabitants of the Australian continent and nearby islands),of mainland Australia, Tasmania, and some of the other adjacent islands.)
Dr David Thomas from the Darwin-based Menzies School of Health Research has stated smoking is the single biggest factor responsible for the gap between the health of Indigenous and non-Indigenous people.
He's called on governments across the country to fund programs aimed at cutting Aboriginal smoking rates. Currently, around 50 percent of indigenous people smoke, compared with just 19 percent of all Australians.
Mr Calma, who'll address an Oceania tobacco control conference in Darwin on Wednesday, says smoking kills one in five indigenous people. Calma: "Indigenous people are more than one-and-a-half times more likely to die from lung cancer than non-indigenous people. Similarly, the much higher death rates from heart disease and other chronic diseases in the indigenous community are in a large part attributable to smoking."
Governments and policy makers should "implement a comprehensive, longer-term national Aboriginal and Torres Strait Islander tobacco control strategy that brings together the various programs and research to drastically reduce smoking rates as soon as possible", Mr Calma said.
The commissioner says he believes mainstream anti-smoking programs need to be made more accessible to indigenous people and tailored to their needs.
The Heart Foundation on Tuesday unveiled its plan to curb smoking among Aboriginal Australians. It's called for specialist tobacco workers to be sent into indigenous communities. The foundation's tobacco spokesman, Maurice Swanson, said much of the difference in life expectancy between indigenous and non-indigenous Australians was due to high rates of cardiovascular and other diseases caused by tobacco.
Mr Calma says smoking is one of the most important health issues facing indigenous people. "If we are to beat this scourge, indigenous people themselves must start to own this issue as a major problem within communities."
A statistical overview of Aboriginal and Torres Strait Islander peoples in Australia..
Reference: Smoking killing Aborigines, says Calma,
Australian Associated Press (AAP), 10/5/2009.
Related news brief: Australia-Northern Territory Medical expert against health care workers that smoke...
Click on images to enlarge, right image Aboriginal and Torres Strait Islander Social Justice Commissioner Tom Calma..
Read more...
Canadian bill to ban flavored tobacco products worries Kentucky burley growers..
 October 6, 2009 - An hour and a half after hearing testimony, a Canadian Senate panel in Ottawa last week approved an anti-smoking bill that Kentucky burley tobacco growers fear may be bad for their business.
October 6, 2009 - An hour and a half after hearing testimony, a Canadian Senate panel in Ottawa last week approved an anti-smoking bill that Kentucky burley tobacco growers fear may be bad for their business.
The bill, known as the Bill C-32 - Cracking Down on Tobacco Marketing Aimed at Youth Act, passed the Senate Social Affairs, Science and Technology Committee on a voice vote and without amendments. It now awaits final action in the Canadian Senate.
Canada’s major health organizations are calling on the Senate of Canada to give priority to the passage of bill. The legislation would stop tobacco companies from using fruit, candy and other flavourings in cigarettes and cigarillos and would ban tobacco ads in publications that can be viewed by youth. C-32 was introduced in the House of Commons by Minister of Health Leona Aglukkaq to implement a commitment made by Prime Minister Stephen Harper. (Canada - federal government introduces legislation to ban flavored tobacco products...
Burley growers are worried that the bill will end the export of American burley to Canada.
We reported on June 17, 2009 that Kentucky tobacco growers contend the Canadian legislation has been written so broadly it could also bar American-blend cigarettes that include burley tobacco. (Canada - Bill C-32 to amend Tobacco Products Control Act..)
Burley is one of three kinds of tobacco mixed together with additives for blended tobacco. Some Kentucky lawmakers, led by Rep. Ed Whitfield, R-1st District, have written to American and Canadian officials that because the pending bill in the Canadian Parliament prohibits many of the additives used in blended tobacco, the measure effectively bans burley.
With 85 percent of U.S. burley exported, the implications of the Canadian action and possible similar actions by other nations are enormous, the Kentuckians warned.
But the Canadian Senate panel did not change any provisions of the bill, despite warnings that the legislation could close the Rothmans, Benson & Hedges cigarette plant in Quebec, where blended tobacco is used.
Debra Steger, an international trade law expert with Rothmans, said that the blended cigarettes in question don’t have a flavored taste like chocolate or fruit — the real target of the legislation. Canada should focus on banning cigarettes that have flavors, not on additives, she said.
But Cynthia Callard, executive director of Physicians for a Smoke-Free Canada, told the Senate committee that the regulations should treat all tobacco products equally and not single out American-style blended cigarettes for special regulation.
An official with Health Canada, the federal government department that deals with health laws, said the new anti-smoking bill would not affect the blended cigarettes that are made in Quebec and then exported.
Reference: Canadian bill worries Kentucky tobacco growers, James R. Carroll (jcarroll@courier-journal.com), Louisville Courier-Journal, 10/3/2009.
Related news briefs: Canadian Tobacco Use Monitoring Survey - 2008..; Canadian House of Commons passes bill to prevent production of mini-cigars or cigarillos..; Canada - Bill C-32 to amend Tobacco Products Control Act..; Canada - federal government introduces legislation to ban flavored tobacco products..; Canada - little cigar/cigarillos smoking declined from 2007 - 2008..; Canadian Cancer Society calls for federal ban on flavored cigars..; Ontario to outlaw candy flavored cigars..; Ontario poised to ban flavored cigarillos..; Canada: a bill introduced to snuff out drive to recruit young smokers..; Still sucking our youngsters in...
Read more...
Cuba - slashes tobacco land, demand for cigars down..
 October 6, 2009 - Cash-short Cuba is slashing the amount of land devoted to growing its famous tobacco by more than 30 percent as the global recession and worldwide spread of smoking bans bite into sales of the country's prized cigars.
October 6, 2009 - Cash-short Cuba is slashing the amount of land devoted to growing its famous tobacco by more than 30 percent as the global recession and worldwide spread of smoking bans bite into sales of the country's prized cigars.
Demand for Cuba's cigars fell 3 percent in 2008 and earlier was reported down 15 percent in 2009 because of the recession and the smoking bans adopted in a growing number of places as a public health measure.
Cuba's National Statistics Office, in a report posted on its web page (www.one.cu), said land to be planted with tobacco for next year's crop had dropped to 49,000 acres, down from 70,000 acres, which was in turn less than 2008.
It said the coming crop was expected to be 22,500 tons, down from a planned 26,800 tons. The office blamed the drop on "financial restrictions that made it impossible to count on the necessary resources."
Cuba's prized cigar brands, including Cohiba, Montecristo, Trinidad and Partagas, dominate the world's premium market with 70 percent of sales.
That jealously guarded market share excludes the United States, however, where Cuba's cigars are banned under the 47-year-old U.S. trade embargo against the communist-led island.
A representative of the exclusive distributor of Cuban cigars, Habanos S.A., a joint venture between Cuba and British tobacco giant Imperial Tobacco Group Plc, said the company had no comment on the statistics office report.
Some 200,000 private farmers and their families depend on growing and curing the precious leaf under contract with the government, and tens of thousands of workers earn their living hand rolling the crop into the famous "Habanos" or "Puros" for export.
Tobacco seedlings are currently being readied for planting from November through January, with harvesting of the quick growing leaf beginning 45 days later. After that a year-long process of drying and curing begins.
Cuba's dozens of cigar rolling factories have operated at well below capacity this year.
Reference: Cuba slashes tobacco acreage amid flagging demand by Marc Frank (Editing by Tom Brown and Padraic Cassidy), Reuters, 10/5/2009.
Related news briefs: Cuba Seeks World Heritage Designation for Cigar-Factory Readers..;
Tampa, FL - Hav-A-Tampa Cigar plant will close at the end of August..;
Habanos - after hurricanes can survive using tobacco reserves..;
Imperial Tobacco Group to Enhance Joint Venture Altadis Had With Habanos Cigars..;
Cuban cigar sales rose 7 percent to $402 million in 2007..;
Altadis (Alliance Tobacco Distributors) born from the merger of Spain's Tabacalera and France's Seita SA (F.STA) state tobacco monopolies...
Read more...
Malaysia - excise duty increased on tobacco - another increase could be coming this month..
 October 6, 2009 - PETALING JAYA: The Government has increased the excise duty on tobacco by 1 sen, or 5.6% per stick, effective last Thursday, though there is no official announcement by the Finance Ministry or Customs Department. (Malaysian ringgit (the Malaysian dollar) is the currency of Malaysia - it is divided into 100 sen. 1 ringlet = 0.2924 USD) (Petaling Jaya commonly called "PJ" by locals) is a Malaysian city originally developed as a satellite township for Kuala Lumpur.) Map of Malaysia..
October 6, 2009 - PETALING JAYA: The Government has increased the excise duty on tobacco by 1 sen, or 5.6% per stick, effective last Thursday, though there is no official announcement by the Finance Ministry or Customs Department. (Malaysian ringgit (the Malaysian dollar) is the currency of Malaysia - it is divided into 100 sen. 1 ringlet = 0.2924 USD) (Petaling Jaya commonly called "PJ" by locals) is a Malaysian city originally developed as a satellite township for Kuala Lumpur.) Map of Malaysia..
The 1 sen per stick duty hike will raise the excise duty per stick to 19 sen. The move caught the industry by surprise as there had been no indication of a tax increase before Budget 2010, which is to be tabled on Oct 23.
Analysts are now expecting cigarette prices to be raised by 1 sen to 2 sen per stick sometime this week. Most industry players are still expecting an increase in taxes for tobacco come Oct 23 as the Government seeks to reduce its deficit.
Terence Wong ... ‘This is the third time the Government has increased excise duty outside the budget period.’ The three main tobacco players in Malaysia are British American Tobacco Malaysia Bhd (BAT), JT International Bhd (JTI) and Philip Morris (M) Sdn Bhd.
The three main tobacco players in Malaysia are British American Tobacco Malaysia Bhd (BAT), JT International Bhd (JTI) and Philip Morris (M) Sdn Bhd.
BAT commands some 70% of the legal cigarette market. When contacted, JTI declined comment. As at press time, its cigarette prices have yet to be raised. BAT, however said it would be raising cigarette prices by 20 sen to 30 sen, effective yesterday.
“Although this is the third time the Government has raised tobacco excise duty outside the budget period, the timing caught us by surprise as we had expected an upward revision to be made only during the Budget 2010 announcement,” said CIMB Research head Terence Wong.
Wong is bracing for another tobacco duty hike this month. Thus, he expects a further 1 sen to 2 sen per stick increase in the upcoming budget, which will bring total excise duty hike for the year to 2 sen to 3 sen per stick.
ECM Libra too felt that a duty hike at this juncture, although unconfirmed, was rather surprising as the tabling of Budget 2010 was only weeks away.
“The small hike suggests that the Government will likely impose another duty hike when Budget 2010 is announced, as it is well known that the Government needs tax revenues to shore up a widening deficit as a result of the fiscal stimulus packages,” it said.
The research house too opined [to state as an opinion] that the Government may impose another duty hike of between 1 sen and 3 sen per stick, thus bringing total duty hike to between 2 sen and 4 sen per stick. In the past few years, the Government has typically raised the excise duty by 3 sen per stick each round.
In the meantime, tobacco players are expected to respond by adjusting their pricing to protect profits. Tobacco players have historically tempered the negative impact of lower industry demand on profits via higher selling prices.
They have traditionally raised prices higher than the quantum of the duty hike to give room for promotional activities needed to cushion the volume drop.
ECM Libra does not expect earnings of tobacco companies to be adversely impacted, unless the quantum of duty hike is beyond its expectations.
Wong forecast a 10% contraction in total industry volume (TIV) from 15.4 billion sticks in 2008 to about 13.8 billion sticks this year. TIV in the first half of 2009 shrank by close to 12% compared with a year ago.
The tobacco sector remained a defensive play and was unlikely to track the market’s upside. “Strong cashflows and attractive yields should provide downside support for these stocks,” Wong said.
References: Government raises excise duty on tobacco by 1 sen, or 5.6% per stick effective Oct 1 - Tobacco industry caught by surprise by YEOW POOI LING and TEE LIN SAY, the Star, 10/6/2009.
Malaysia - related news briefs:
Malaysia - smoking limits your quality of life..;
Malaysia - smoking civil servants in Penang to receive free nicotine treatment..;
Malaysia - illicit cigarettes, BAT wants government to slow excise duty increases..;
Peninsular Malaysia - one of three cigarette packs is either contraband or fake..;
Komtar, Penang, Malaysia smoking ban strictly enforced..;
Malaysia student forced to smoke 40 cigarettes in two hours..
Malaysia - PSD and Cuepacs are at odds over the no-smoking rule at government departments and agencies..;
Malaysia: Are tobacco control measures working? - WHO thinks so...;
Malaysia - slowdown in cigarette consumption..;
Malaysia - January 1, 2009 pictorial cigarette warnings..;
Malaysia to hike cigarette prices..;
Malaysia - 25% of all cigarettes sales are illegal...Peninsular Malaysia - one of three cigarette packs is either contraband or fake..;
Komtar, Penang, Malaysia smoking ban strictly enforced..;
Malaysia student forced to smoke 40 cigarettes in two hours..
Malaysia - PSD and Cuepacs are at odds over the no-smoking rule at government departments and agencies..;
Malaysia: Are tobacco control measures working? - WHO thinks so...;
Malaysia - slowdown in cigarette consumption..;
Malaysia - January 1, 2009 pictorial cigarette warnings..;
Malaysia to hike cigarette prices..;
Malaysia - 25% of all cigarettes sales are illegal...
Read more...


To Provide Public Awareness
Purpose
About Us
Contact Us
2008 HIGHLIGHTS
TOPIX PAPERS - 2008 & 2009..
Archive
-
▼
2009 (1446)
-
▼
10/04 - 10/11 (34)
- FDA says delaying tobacco authority will harm publ...
- Croatia - lifted smoking ban in bars and cafes..
- Nevada - State Supreme Court upholds smoking ban, ...
- Australian Health Minister - orders facebook probe..
- Canada - bill to ban flavored tobacco products ge...
- Canada - bill to ban flavored tobacco products get...
- RAI to webcast q3 2009 earnings conference call on...
- United Kingdom - limiting access to cigarette ven...
- R.J. Reynolds - names like 'Light' 'Ultra-Light...
- Juneau, Alaska - voters say yes to raising tobacco...
- FDA listening sessions for tobacco stakeholders..
- San Francisco - Michael Jordan caught smoking on p...
- Nat Sherman Co. letter clarifying company's cigare...
- South Dakota - statewide smoking ban trial date ...
- U.S. Institute of Medicine: Report - Secondhand-...
- Altria to Host Webcast of q3 2009 results on Octob...
- Social networking sites marketing tobacco product...
- Nicotine Vaccine - funding for Phase 3 clinical tr...
- Smoking during pregnancy and postnatal environment...
- Wales - Smoking 'costs NHS £1m (1.6m USD) each da...
- New Jersey assemblywoman wants to limit electroni...
- Australia - heavy tobacco use killing Aborigines..
- Canadian bill to ban flavored tobacco products wor...
- Cuba - slashes tobacco land, demand for cigars d...
- Malaysia - excise duty increased on tobacco - anot...
- Ontario Campaign for Action on Tobacco (OCAT) - ne...
- Quebec - intends to sue cigarette makers to recoup...
- Bulgaria and others - smoking ban, increased ciga...
- Ireland - Philip Morris and retailer to file lawsu...
- For our children ban all flavored tobacco products..
- Bavaria - court rules, looser implementation of sm...
- Finland - government proposing ban on smoking when...
- US - National Breast Cancer Awareness Month, what ...
- India - one year later, most states yet to implem...
-
▼
10/04 - 10/11 (34)