 September 10, 2010 - The U.S. Food and Drug Administration (FDA) today, September 9th issued warning letters to five electronic cigarette distributors for various violations of the Federal Food, Drug, and Cosmetic Act (FDCA) including unsubstantiated claims and poor manufacturing practices. ‘The companies receiving warning letters today [Thursday] are: E-CigaretteDirect LLC, Ruyan America Inc., Gamucci America (Smokey Bayou Inc.), E-Cig Technology Inc. and Johnson Creek Enterprises LLC.’
September 10, 2010 - The U.S. Food and Drug Administration (FDA) today, September 9th issued warning letters to five electronic cigarette distributors for various violations of the Federal Food, Drug, and Cosmetic Act (FDCA) including unsubstantiated claims and poor manufacturing practices. ‘The companies receiving warning letters today [Thursday] are: E-CigaretteDirect LLC, Ruyan America Inc., Gamucci America (Smokey Bayou Inc.), E-Cig Technology Inc. and Johnson Creek Enterprises LLC.’ Also today, in a letter to the Electronic Cigarette Association, FDA said the agency intends to regulate electronic cigarette and related products in a manner consistent with its mission of protecting the public health. The letter outlines the regulatory pathway for marketing drug products in compliance with the FDCA.
Also today, in a letter to the Electronic Cigarette Association, FDA said the agency intends to regulate electronic cigarette and related products in a manner consistent with its mission of protecting the public health. The letter outlines the regulatory pathway for marketing drug products in compliance with the FDCA.
See the FDA Release (links provide a lot of useful information): FDA acts against 5 electronic cigarette distributors
Background: The FDA in February 2010 won a temporary delay of a U.S. judge’s ruling that the agency lacks authority to regulate the products as drugs or medical devices and must allow e-cigarettes to be imported. A panel of three appeals court judges temporarily stayed the lower court ruling in order to give them more time to consider the FDA's arguments.For a drug product to gain FDA approval, a company must demonstrate to the agency that the product is safe and effective for its intended use. The company must also demonstrate that manufacturing methods are adequate to preserve the strength, quality and purity of the product. But is an e-cigarette a drug.... We agree with policy adopted on June 14, 2010 by the American Medical Association (AMA) that recommends that electronic cigarettes be classified as drug delivery devices, subject to the same FDA regulations as all other drug delivery devices. Additional policy adopted supports prohibiting the sale of e-cigarettes that are not FDA approved. (US AMA policy - e-cigarettes FDA should treat as a drug delivery device..)
ORAL ARGUMENT SCHEDULED FOR SEPTEMBER 23, 2010
NO. 10-5032
IN THE UNITED STATES COURT OF APPEALS
FOR THE DISTRICT OF COLUMBIA CIRCUIT
SMOKING EVERYWHERE, INC.,
SOTTERA, INC., d/b/a NJOY,
Intervenor-Plaintiff-Appellee,
v.
U.S. FOOD AND DRUG ADMINISTRATION, et al.,
Defendants-Appellants.
On Appeal From the United States District Court For the District of Columbia
Civil Action No. 09-cv-0771 (RJL)
Directly related news brief: In Process: US. Federal District court judge makes serious error regarding the import of e-cigarettes..; U.S. FDA - judges ruling regarding e-cigarettes must be appealed - ASAP..; U.S. FDA appealing a federal judge's ruling on e-cigarettes.; U.S. - federal appeals court, import of e-cigarettes on hold again..; THIS MUST STOP - e-cigarette company asking active duty soldiers to buy their product..; ; U.S. e-cigarette imports banned indefinitely - ASH...
Michael Levy, director of the agency’s Division of New Drugs and Labeling Compliance: “Although these products are frequently marketed to help consumers quit smoking, the FDA has not evaluated them for safety or effectiveness.”
Click to enlarge..
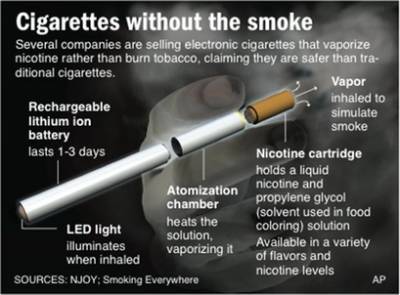 E-cigarettes are smokeless devices that deliver nicotine to the user. They consist of three integrated parts: the nicotine cartridge, the vaporizer and a lithium ion battery. The battery powers the cartridge and releases the nicotine by heating, rather than burning like a conventional cigarettes. They are available in fruit and candy flavors. Little independent research has been conducted into their ingredients and health impacts, but they are commercially promoted by vendors as a safe alternative to cigarettes.
E-cigarettes are smokeless devices that deliver nicotine to the user. They consist of three integrated parts: the nicotine cartridge, the vaporizer and a lithium ion battery. The battery powers the cartridge and releases the nicotine by heating, rather than burning like a conventional cigarettes. They are available in fruit and candy flavors. Little independent research has been conducted into their ingredients and health impacts, but they are commercially promoted by vendors as a safe alternative to cigarettes.References: FDA Sends Warning Letters to Electronic Cigarette Makers by MELLY ALAZRAKI, Daily Finance, 9/9/2010; E-Cigarettes Violate Drug, Device Rules, FDA Says by Molly Peterson (Editors: Neil Gross, Jeffrey Tannenbaum), Bloomberg Businessweek, 9/9/2010.


2 comments:
September 16, 2010 at 12:38 AM
I think FDA is just doing their job. This a good move so that companies would comply with FDA regulations.
e cigarette reviews
October 30, 2012 at 11:57 AM
Post a Comment