
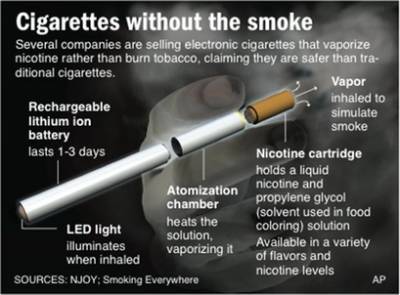
 June 28, 2010 - In advance of the state's smoking ban on July 5, the Brown County Tavern League is selling electronic cigarettes, battery-powered devices that use liquid nicotine to imitate a cigarette's taste and effects is selling electronic cigarettes, battery-powered devices that use liquid nicotine to imitate a cigarette's taste and effects. (Green Bay, WI is in Brown County)
June 28, 2010 - In advance of the state's smoking ban on July 5, the Brown County Tavern League is selling electronic cigarettes, battery-powered devices that use liquid nicotine to imitate a cigarette's taste and effects is selling electronic cigarettes, battery-powered devices that use liquid nicotine to imitate a cigarette's taste and effects. (Green Bay, WI is in Brown County)
The league began selling the devices in March, and the demand from bars and taverns around the state has been "crazy," said Brown County Tavern League President Sue
The Tavern League of Wisconsin is a non-profit trade association dedicated to serving the needs of the retail beverage alcohol segment of the hospitality industry in the State of Wisconsin. (Tavern League of Wisconsin)
Brown County Tavern League President Sue Robinson, "We're hoping that it'll keep our smoking customers comfortable and coming to our business," said Robinson, who sells the devices through her tavern.
Electronic cigarettes - study finds not an effective nicotine delivery system..
Units can cost more than $100, but the league charges $60 each because Robinson said the organization buys directly from a distributor called AirE8. The league also sells 10 refills of nicotine for $15. One refill of nicotine can equal one-and-a-half packs of normal cigarettes, which is less expensive than a pack of cigarettes, the cheapest being $5.59, without tax.
We do not understand why these e-cigarette devices are being sold in the US. The importation of e-cigarettes was banned indefinitely as the result of a unanimous ruling by the U.S. Court of Appeals. (U.S. - federal appeals court, import of e-cigarettes on hold again..)
Just recently, on June 14th by the American Medical Association (AMA) recommended that electronic cigarettes (e-cigarettes) be classified as drug delivery devices, subject to the same FDA regulations as all other drug delivery devices. The AMA supports prohibiting the sale of e-cigarettes that are not FDA approved. (US AMA policy - e-cigarettes FDA should treat as a drug delivery device..)
In order for any drug or drug delivery device to be marketed in the U.S., it must first be approved by the FDA. To gain FDA approval, the company intending to market a specific drug must conduct clinical tests to demonstrate that the drug is both safe for use and that it works for the purpose for which it is intended. Once clinical testing is complete, the results are presented to an FDA panel of experts for evaluation. If the panel believes the clinical test results demonstrate both safety and efficacy, the drug is recommended for approval. (e-cigarettes - FDA approval needed prior to marketing..)
The devices have been banned inCanada and Australia.
Reference: Brown County Tavern League sells e-cigarettes as alternative after ban by Ilissa Gilmore (igilmore@greenbaypressgazette.com), GreenBaypressGazette.com, 6/27/2010.


0 comments:
Post a Comment